Carbohydrates are the biomolecules consisting of aldehyde and ketone with multiple -OH groups. Carbohydrates are the main source of energy are made of 3 elements Carbon, Hydrogen, and Oxygen some of them also contain phosphorus or Sulphur.
Classification of carbohydrates
Carbohydrates are mainly classified as :
- Monosaccharides
- Oligosaccharides
- Polysaccharides
The word saccharide derived from the Greek word sakcharon referring to “Sugar”.
Monosaccharides:
- Monosaccharides are the simplest of all the carbohydrates also known as simple sugar. These are the building block for complex carbohydrates. The most abundant simple sugar found in nature is the six-carbon sugar D-glucose, also called dextrose. Monosaccharides family starts with 3 carbon to 7 carbons backbones. Most of the monosaccharides have the chemical formula (CH2O)n. (n is the no of carbon atom present)
- Examples: Glucose, galactose, fructose, ribose
- Dihydroxyacetone acetone and L-glyceraldehyde is the smallest monosaccharides.
- Monosaccharides are crystalline solids, colorless, and freely soluble in water but insoluble in non-polar substances. Most of the monosaccharides have a sweet taste. E.g. Glucose
- The symbols D and L designate the absolute configuration of the asymmetric carbon which is farthest from the aldehyde or keto group.
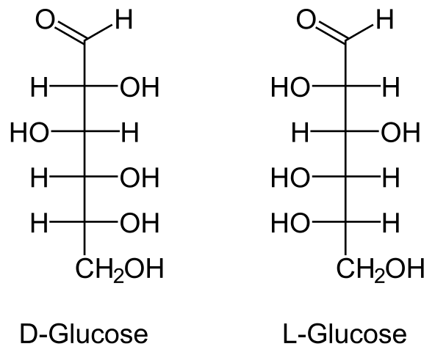
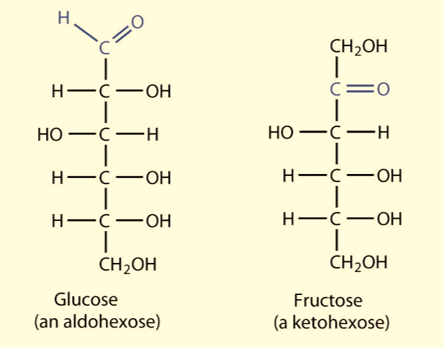
- If the carbonyl group of the sugar is attached at the end of the carbon chain the monosaccharide is aldose and if the carbonyl group is at any other position, then the monosaccharide is a ketose.
- Monosaccharides having 3 carbon backbone are called Triose and four, five, six, and seven carbon backbone are called Tetroses, Pentose, Hexoses, Heptoses
- Glucose is the most important monosaccharide. Glucose is used in our body for energy production. Excess glucose (Glycogen) is stored in the liver and muscle cells. Excess glucose in our body is converted to fat and stored as adipose tissue.
- Galactose is present in many plant gums and pectins and a component of disaccharide lactose.
- Fructose is generally present in fruits, honey. It is the sweetest of all occurring sugars.
- Most of the sugars are present in D form in nature except L-arabinose.
- Monosaccharides are linked to alcohols and amines through glycosidic bonds.
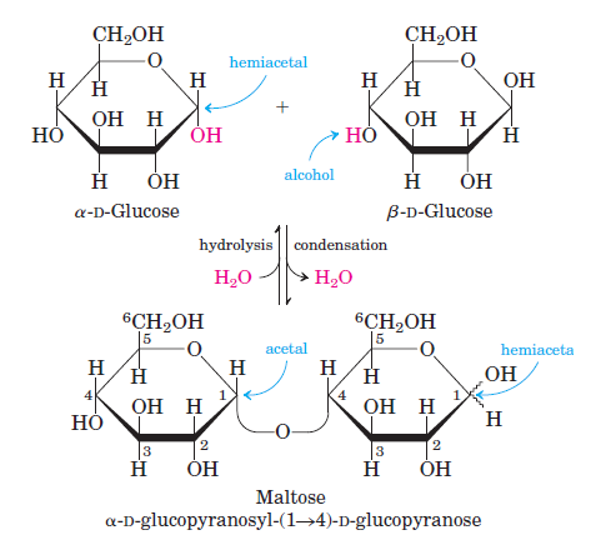
- Monosaccharides have cyclic forms. The formation of the cyclic ring is the result of a reaction between alcohol and aldehyde/ketone groups to form derivatives that are called Hemiacetals/Hemiketals.
- Five-member ring compounds are called furanoses and six members ring compounds are called Pyranoses.
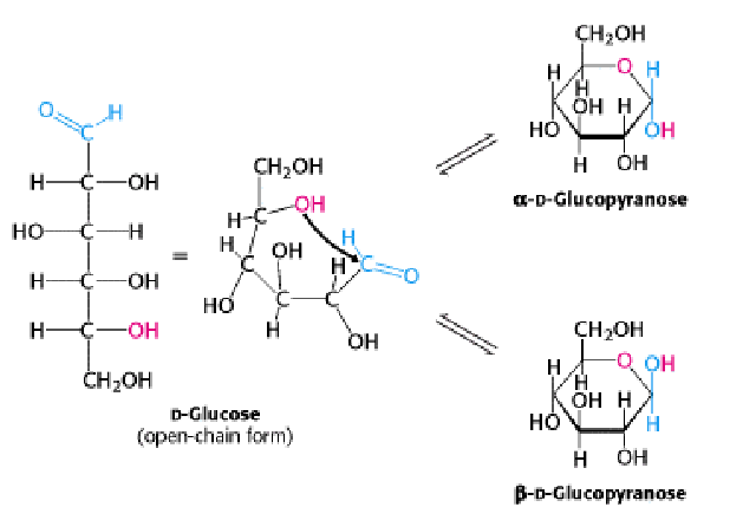
Pyranose Formation:
The open chain of glucose gets converted into its cyclized structure when the C-5 hydroxyl group attacks the oxygen atom of the C-1 aldehyde group to form an intramolecular hemiacetal. Two anomeric forms, designated α, and β can result. An anomeric carbon is a carbon derived from the carbonyl carbon of the open-chain form of the carbohydrate molecule.
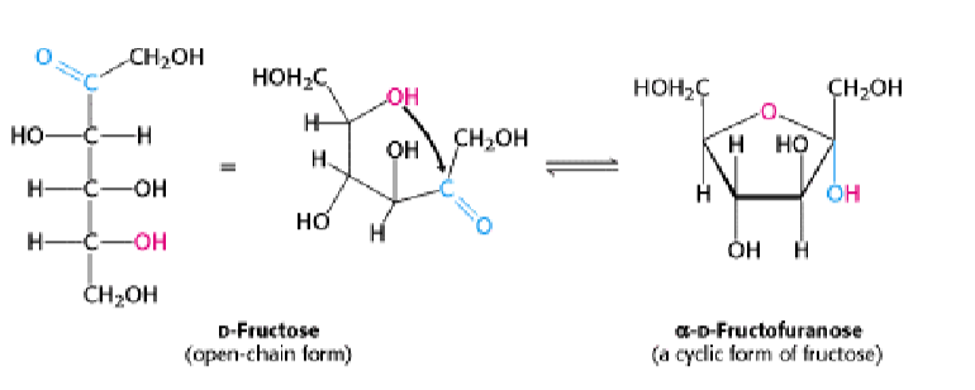
Furanose Formation:
The open chain form of fructose gets converted into its cyclized form (to a five-membered ring) when the C-5 hydroxyl group attacks the C-2 ketone to form an intramolecular hemiketal. Two anomers are possible, but only the α anomer is shown.
Stereoisomers:
Two isomeric molecules having similar formula but differ in the three-dimensional orientation of their atoms in space.
‘A molecule with n chiral centers can have 2n stereoisomers’(Fisher projection)
Glucose has 4 chiral carbons and 16 stereoisomers.
Enantiomers:
d-Glyceraldehyde and l-glyceraldehyde are enantiomers or mirror images of each other.
Diastereoisomers:
These are a class of stereoisomers that are not mirror images of each other.
Epimers:
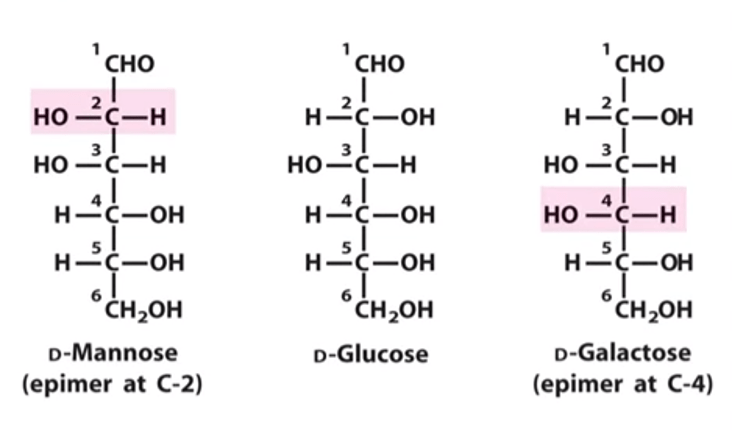
Sugars differing in configuration at a single asymmetric center are called epimers. Thus, d-glucose and d-mannose are epimeric at C-2; d-glucose and d-galactose are epimeric at C-4.
Disaccharides:
Disaccharides are consisting of 2 monosaccharides joined covalently through an O-glycosidic bond that is formed when a -OH group of sugar reacts with anomeric carbon of the other.
Examples:
- Lactose: Glucose and galactose
- Maltose: glucose and glucose
- Sucrose: glucose and fructose
Oligosaccharide is the polysaccharide of low molecular weight. A polymer of 3-8 monosaccharide units.
Polysaccharide:
carbohydrates consisting of large numbers of monosaccharides units linked through glycosidic bonds.
Examples:
- Starch: Part of the human diet, found in potato, rice, and cereal grain. Starch is polymer os amylose and amylopectin.
- Glycogen: stored glucose in the body in the liver and muscle.
- Cellulose: a structural component of the plant cell wall. Humans and animals can’t digest cellulose because our digestive systems don’t content beta-glucosidase enzymes that catalyze the hydrolysis of beta glucosidic bonds.
References:
- Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2005). Lehninger principles of biochemistry. Macmillan.
- Berg, J. M., Tymoczko, J. L., & Stryer, L. (2002). Monosaccharides are aldehydes or ketones with multiple hydroxyl groups. Biochemistry, 5th edition: New York.